Pharmaceuticals
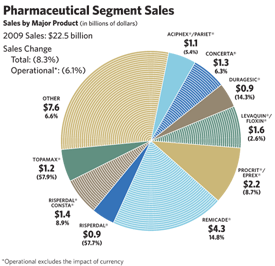
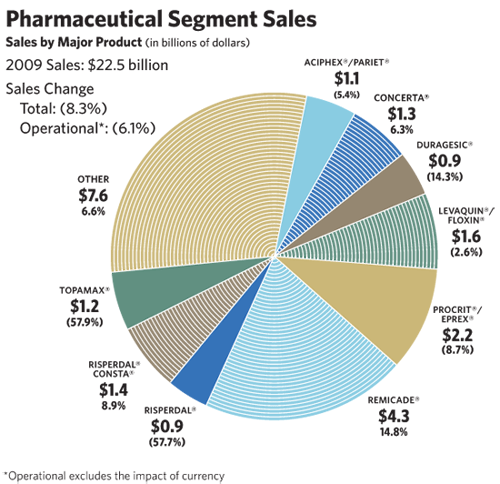
Our Pharmaceuticals segment, with sales of $22.5 billion, represents the world’s seventh largest pharmaceutical business and fourth largest biotech business. The segment experienced an operational sales decline of 6.1 percent in 2009, reflecting the loss of nearly $3 billion in sales due to losing market exclusivity for RISPERDAL® (risperidone) and TOPAMAX® (topiramate). Excluding the impact of generic competition, pharmaceutical sales increased by approximately 7 percent
operationally.
This growth was driven by larger products, including REMICADE® (infliximab), for the treatment of a number of immune-mediated inflammatory diseases; CONCERTA® (methylphenidate HCl) Extended-release Tablets in attention deficit hyperactivity disorder (ADHD); and RISPERDAL® CONSTA® (risperidone) Long-Acting Injection, an atypical antipsychotic administered every two weeks for the treatment of schizophrenia or the maintenance of bipolar 1 disorder. Promising newer products continued their positive growth trajectory, such as PREZISTA® (darunavir) in HIV; VELCADE® (bortezomib), for multiple myeloma, developed in partnership with Millennium: The Takeda Oncology Company (we have rights outside the U.S.); INVEGA® (paliperidone), a once-daily atypical antipsychotic for the treatment of schizophrenia or acute schizoaffective disorder; and INTELENCE™ (etravirine), for HIV combination therapy.
Our pharmaceutical pipeline is one of the most robust in our history. We launched five newly approved drugs in 2009: SIMPONI™ (golimumab) and STELARA™ (ustekinumab) in immunology; NUCYNTA® (tapentadol) Immediate Release Tablets for pain relief and INVEGA® SUSTENNA™ (paliperidone palmitate) for the treatment of schizophrenia; and PRILIGY™ (dapoxetine) in select countries across the world in sexual health. In addition, we continue to expand our core products with new indications, a practice we have done well historically. For example, REMICADE® now has 15 FDA-approved indications across a broad spectrum of immune system disorders.
Our future pipeline is promising. An important product in registration is rivaroxaban, which we are co-developing with Bayer HealthCare AG. Rivaroxaban is a novel oral anticoagulant that may prevent a host of thrombotic conditions, including venous thromboembolism and stroke in atrial fibrillation. It is being evaluated in five different indications. And we have important compounds in Phase III clinical trials, including treatments for diabetes, prostate cancer and Alzheimer’s disease.
Building on our already strong pipeline, we engaged in acquisitions and innovative agreements and collaborations with companies that offer potentially significant advances in patient care. These include a potential first-in-class treatment for slowing the progression of Alzheimer’s disease (Elan Corporation, plc); a potential universal monoclonal antibody product for the treatment and prevention of influenza (Crucell NV); an HIV therapy with a single combination pill (Gilead Sciences, Inc.); and a potential breakthrough in prostate cancer (Cougar Biotechnology, Inc.).
While strengthening its pipeline, our Pharmaceuticals business expanded geographically, with a focus on emerging markets. We have been expanding our sales reach in China; maintaining a strong manufacturing footprint in China, Mexico and Brazil; and developing our R&D presence in emerging markets like India and China by establishing an R&D operation in Mumbai, an R&D headquarters in Shanghai and a collaboration with Tianjin Medical University Cancer Hospital on biomarker research.