Medical Devices and Diagnostics
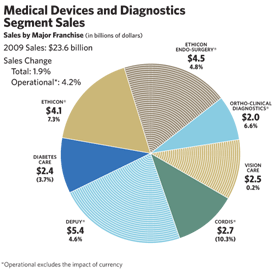
The Medical Devices and Diagnostics (MD&D) franchises comprise the world’s largest medical technology business, with 2009 sales of $23.6 billion, an increase of 4.2 percent operationally. Four of the seven franchises had solid sales gains during the past year. Tougher competition for drug-eluting stents and tighter out-of-pocket spending on products like contact lenses and diabetes test strips pressured sales in our Cordis Corporation, Diabetes and Vision Care franchises.
Growth products spanned a range of treatment categories, including wound care products and biosurgicals from Ethicon, Inc.; energy technology and the REALIZE® Adjustable Gastric Band-C from Ethicon Endo-Surgery, Inc.; artificial joints, spine and sports medicine products from DePuy, Inc.; and new products from Ortho-Clinical Diagnostics, Inc.
Several products introduced new standards of care for the medical devices industry. CARTO® 3, from Biosense Webster, Inc., gives physicians a detailed three-dimensional view of the heart so they can treat cardiac arrhythmias, including atrial fibrillation.
The SURGIFLO® Hemostatic Matrix Kit, our advanced flowable hemostat for use in a broad range of surgical procedures, is the first product launch from the acquisition of Omrix Biopharmaceuticals, Inc. and an example of technology resulting from the combination of our medical device and biologics expertise.
Our Vision Care franchise continued the global rollout of 1·Day ACUVUE® TruEye™, the world’s first daily disposable silicone hydrogel contact lens and an exciting breakthrough in contact lens technology. We anticipate introduction in the U.S. in 2010.
MD&D also strengthened its portfolio through several recent strategic acquisitions. These included Acclarent, Inc. in the ear, nose and throat surgical space; Finsbury Orthopaedics, Ltd. in hip implants; and Gloster Europe, a developer of innovative area-decontamination technologies to help prevent health care-acquired infections, a growing global concern.
The pipeline is strong with promising new products such as SEDASYS® System, the first computer-assisted personalized sedation system, and the PINNACLE® CoMplete™ Acetabular Hip System, the first ceramic-on-metal hip replacement. Both products received favorable recommendations from U.S. Food and Drug Administration (FDA) Advisory Committees in 2009.
In addition to new product introductions and robust pipelines, MD&D continually expanded its global reach, particularly in emerging markets, with research and development centers, professional training centers and manufacturing facilities.